Interfacial Mechanochemistry of Biological Adhesives
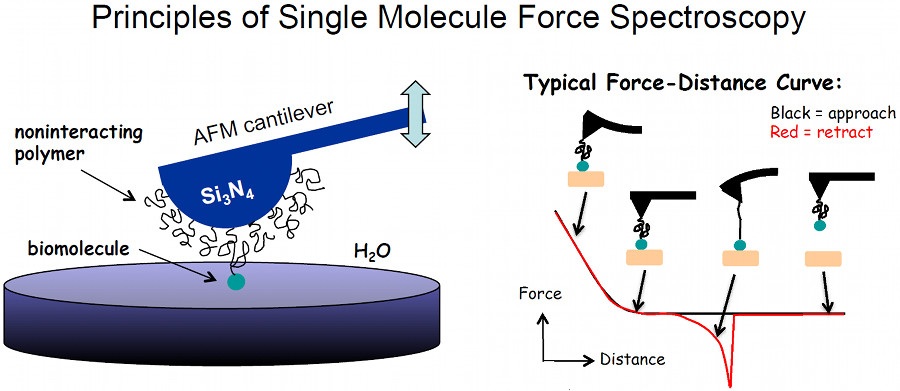
We are using single molecule force spectroscopy to probe the interaction of adhesive biomolecules with surfaces (see Figure). In this method, and atomic force microscope (AFM) cantilever is functionalized with an inert grafted polymer with a small fraction of grafted polymers terminally functionalized with the biomolecule of interest (e.g. amino acid, peptide, protein). The cantilever is then brought into contact with a surface and pulled away to measure the force necessary to rupture the bond linking the biomolecule to the surface. The force required to dissociate the biomolecule can range from a few tens of pN to over 1 nN and reflects the nature of the chemical interaction.
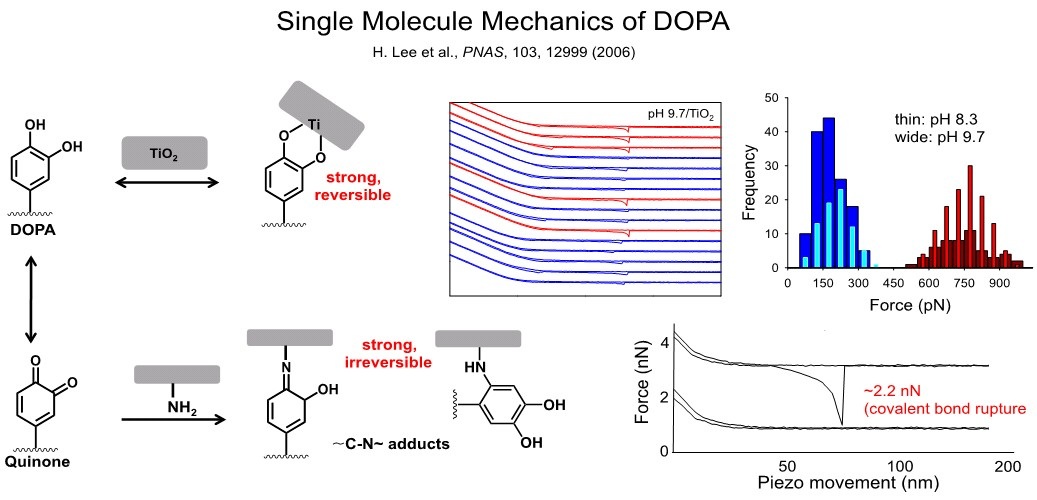
We have used this method to investigate the interaction of DOPA amino acid with organic and surfaces under conditions relevant to mussel protein adhesion (Figure). Our studies revealed that DOPA interacts strongly and reversibly to inorganic oxide (TiO2), suggesting an important interfacial adhesive role for DOPA in mussel proteins. Interestingly, tyrosine amino acid, from which DOPA is derived, interacts only weakly with oxide surfaces, implying that hydroxylation of tyrosine is important to adhesion. In this study we also gained an appreciation for the chemical versatility of DOPA, as force measurements performed on nucleophilic organic surfaces under alkaline pH conditions revealed the formation of covalent bonds due to C-N bond formation between DOPA and amine functional groups on the organic surface. Taken together, these experiments suggest an immense versatility in adhesive chemical interactions that DOPA can undertake with inorganic and organic surfaces.