We are developing novel hydrogels and other materials for use in regenerative medicine and general surgery. These hydrogels frequently are designed to solidify in-situ for bonding of tissues together, for attaching medical devices to tissues, and for sealing wounds or surgically created defects in tissue membranes. Often we take advantage of mussel-inspired chemistry to form cross-links and for adhesion to tissue surfaces. Several examples are described below.
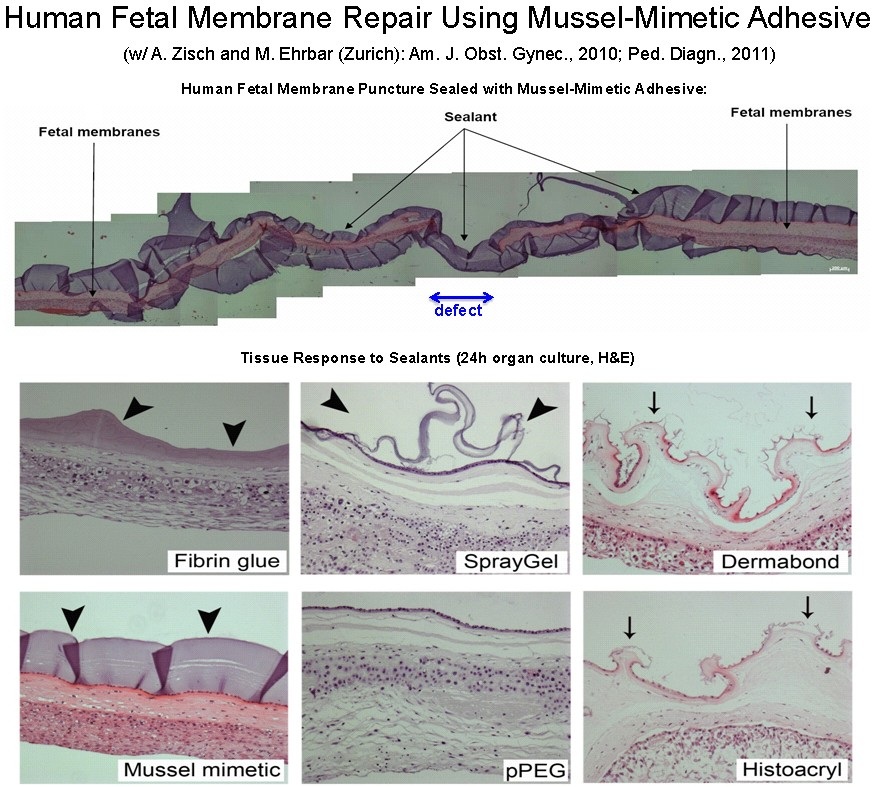
One frontier in the medical use of hydrogels is in fetal surgery, a growing surgical specialty concerned with performing interventional procedures on the fetus while it is in the womb. This is a particularly challenging environment for an adhesive due to the large volume of aqueous fluid, the confounding effects of water on adhesion, and complicated surgical access. There is a great need for novel materials that can be used as sealants and adhesives in fetal surgery. One compelling example is the sealing of the fetal membranes (more commonly known as the amniotic sac). The fetal membranes have limited ability to heal from spontaneous or surgically created ruptures, yet suturing is challenging and of limited success in preventing amniotic fluid leakage. We have explored the use of our mussel inspired gels for sealing of fetal membranes. As shown in the figure, mussel inspired hydrogels successfully sealed puncture wounds of fresh human fetal membranes, exhibiting good adhesion to the tissue and good acute biocompatibility in a 24 hour organ culture model experiment
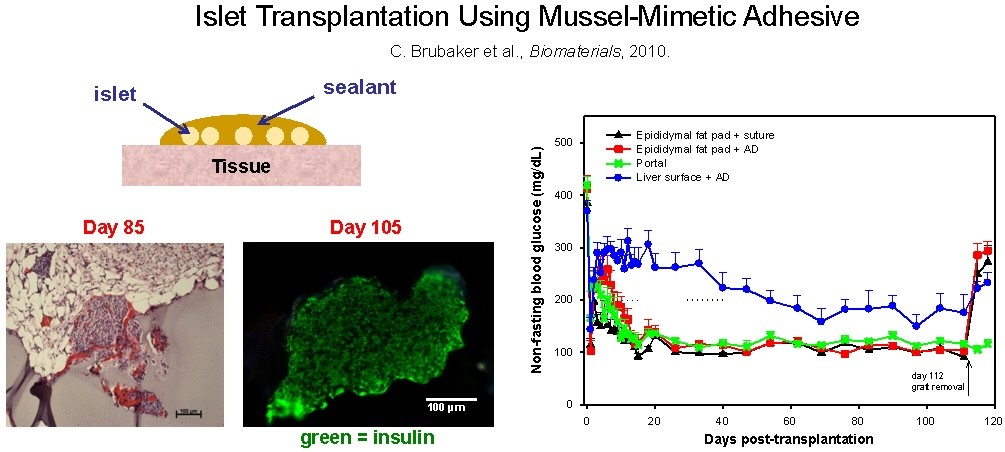
We have explored the use of catechol derivatized poly(ethylene glycol) (PEG) for islet transplantation. Here, the gel is meant to facilitate minimally invasive transplantation of islets by bonding islets onto a tissue surface by in-situ formation of a conformal gel coating. Shown in the figure is an image of an islet bound to the surface of tissue by a mussel inspired gel. The islets were revascularized from the underlying tissue and managed blood glucose concentration. Animals treated in this manner returned to euglycemia within 5-10 days after transplantation.
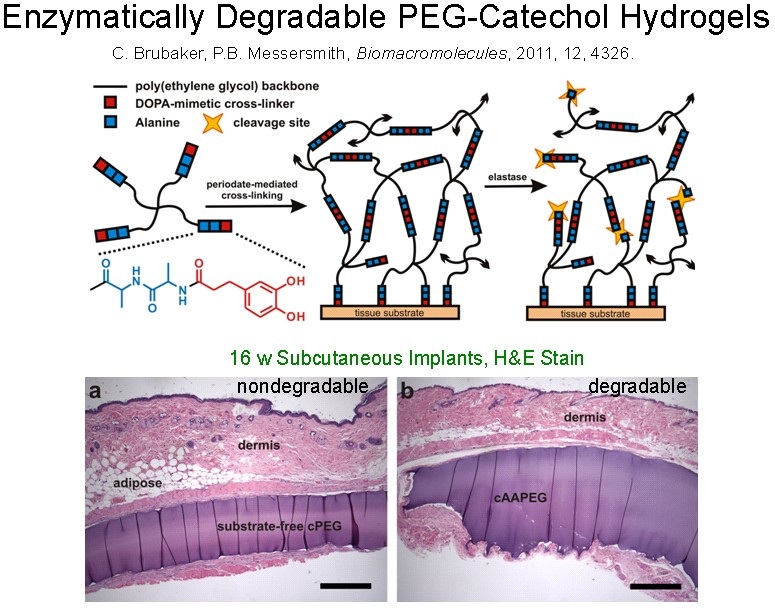
If desired, degradability in these hydrogels can be introduced in a variety of ways including the introduction of hydrolysable or enzymatically cleavable linkers. In this figure, an enzymatic strategy is depicted. An alanine dipeptide linker provides a mechanism for degradation through its ability to be cleaved by human neutrophil elastase.